How to Write Ionic Equations
A redox reaction is the one in which oxidation and reduction occur. Theres a particular way of writing whats in a molecule called a chemical formula.

How To Write Ionic Equations Tutor Pace Science Chemistry Chemistry Class Chemistry Notes
It is a set of nonlinear differential equations that approximates the electrical characteristics of excitable cells such as neurons and muscle cells.

. For calculations clearly show the method used and the steps involved in arriving at your answers. These electrolytes are split up and written as individual ions when written in ionic chemical equations. Ionic chemical equations are slightly different from that of a classic case of chemical equations.
B Identify and write out all redox couples in reaction. Here are the steps involved in balancing chemical equations plus a worked example. Being able to balance chemical equations is a key chemistry skill.
In aqueous solutions its common to balance chemical equations for both mass and charge. Write the equation and the. Write the molecular total ionic and net ionic equations illustrating the reaction.
The quantities of substances produced or consumed by the electrolysis. Here are the steps involved in balancing chemical equations plus a worked example. The chemical formulae for all the elements that form each molecule and uses a small.
For the reaction of magnesium with hydrochloric acid is. It is a continuous-time dynamical system. The complete Maxwell equations of electrodynamics give us much more information although even then the answer is strictly speaking not unique We will therefore discuss this question in detail again in a later chapter.
We usually write ionic equations to show precipitation reactions. Methanoicacid HCOOH ionizes according. Spectator ions are ions that are Not changing state Not changing oxidation number Take full equation BaNO32 aq Na2SO4 aq BaSO4 s 2 NaNO3 aq Separate aq solutions into ions Ba2 aq 2NO3.
K sp 0015900318 2 161 x 10-5. A solution of AgNO 3 is mixed with a solution of K 2 S. Examples and equations may be included in your responses where appropriate.
A greater chance of. S for a solid l for a liquid g for a gas and aq for an aqueous solutionThis is especially done when one wishes to emphasize the states or changes thereof. More collisions per second is fine though.
This applies to both A-level and GCSE. It is not enough to say only there are more collisions as there is no reference to time. Modification of work by vxlaFlickr.
Calculating the Solubility of an Ionic Compound in Pure Water from its K sp. The ionic equations can be represented by two half equations. Being able to balance chemical equations is a key chemistry skill.
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. 2H aq Mgs Mg 2 aq H 2 g This ionic equation can be split into two half equations. Modification of work.
Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current. Guidelines for balancing redox equations. Example of Balanced Ionic Equation.
This worksheet will help you practise writing ionic equations for neutralisation and precipitation reactions Where state symbols are not given youll need to use the solubility rules to determine whether a substance will ionise Write ionic equations for the following. A 18 10 4. Write balanced formula unit total ionic and net ionic equations for the reaction Question 14.
Science Tech Math Science Math Social. Explain why this is a redox reaction. HNO 3aq NaOH aq NaNO 3aq H 2 O l 2.
Ionic chemicals involve electrolytes which are substances that dissociate into ions when dissolved in polar solvents. Write an unbalanced equation. We will give you now only the result for the particular case of electrostatics.
2 l H O3 aq HCOO. Write a balanced half equation for the formation of calcium from a calcium ion Ca 2. For example the reaction of aqueous hydrochloric acid with solid metallic sodium to form.
For example the ionic equation. Separate the process into half reactions a Assign oxidation numbers for each atom. Alan Hodgkin and Andrew Huxley described the model in 1952 to explain the ionic mechanisms underlying the initiation and propagation of action potentials in the squid giant axon.
Pay attention to significant figures. It is amazing how many marks are thrown away when writing about the collision theory. The two halves of the ionic equation representing one at cathode and another at anode areZnto Zn22e- 2H2e-to H_2 iii.
Increasing temperature concentration and surface area all increase the frequency of collisions. So chemists use symbols just like its done in maths. The energy is located in space where the electric field is.
Fourth substitute the equilibrium concentrations into the equilibrium expression and solve for K sp. You must show your work to receive credit for your answer. Ca 2 2e- Ca.
Chemists have to write chemical equations all the time and it would take too long to write and read if they had to spell everything out. Balancing for mass produces the same numbers and kinds of atoms on both sides of the equation. Ionic equations only show the ions that are reacting and leave out spectator ions.
Chemistry - how to write balanced ionic equations Molecular Complete Ionic and Net Ionic Equations How to write ionic and net ionic equations How to write a double replacement net ionic equation what are spectator ions precipitation reaction single displacement reaction with video lessons examples and step-by-step solutions. Each element has a symbol. The ions are forced to undergo either oxidation at the anode or reduction at the cathode.
To indicate physical state of a chemical a symbol in parentheses may be appended to its formula. Balance the atoms in each half reaction a Balance all other atoms except H. C Combine these redox couples into two half-reactions.
The state of matter aq stands for aqueous. Question 15Write decomposition reactions for the following compounds aCaHCO 3 2 bAg 2 O cN 2 O 3 Question 16Write one equation each for. Estimate the solubility of Ag 2 CrO 4 in pure water if the solubility product constant for silver chromate is 11 x 10-12.
Electrolysis involves passing an electric current through either a molten salt or an ionic solution. These are half equations for some reactions where negatively charged ions. Modification of work by the Italian voiceFlickr.
Balancing for charge means the net charge is zero on both sides of the equation.

Net Ionic Equation Worksheet And Answers Youtube Equations Chemistry Study Tips

Net Ionic Equations Ap Chem Chemistry Class Equations
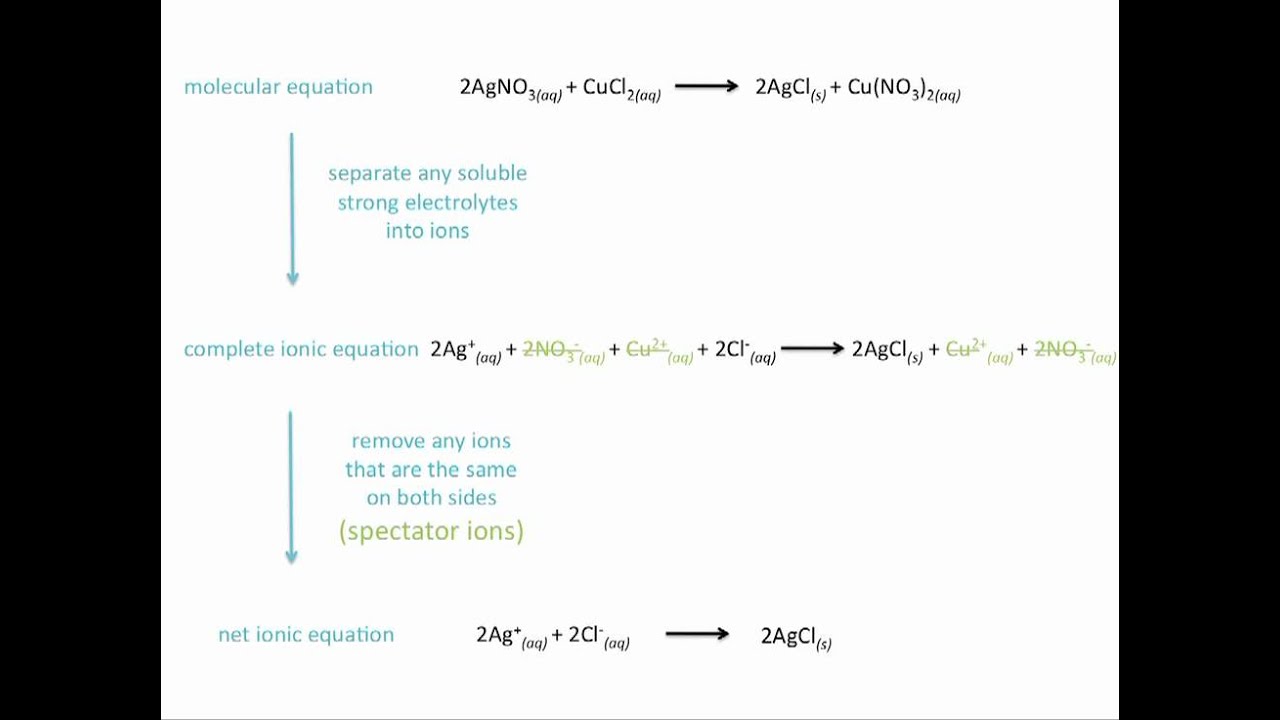
Ionic Equations Net Ionic Equations And Spectator Ions Chemistry Tutorial Chemistry Equations Ionic

How To Write The Net Ionic Equation For Hcl Zns H2s Zncl2 Chemical Equation How To Find Out Math

Comments
Post a Comment